Brian Gac, Hanna D. Yakubi and Dorie E. Apollonio
This blog post is based on the Evidence & Policy article, ‘Issues arising from the study design, conduct, and promotion of clinical trials funded by opioid manufacturers: a review of internal pharmaceutical industry documents’.
From 1999 to 2021, opioid overdoses caused over one million deaths in the US. The pharmaceutical industry has been held legally responsible in some cases for overstating the benefits and understating the risks of opioid use, leading to overprescribing that contributed to these deaths. Opioid manufacturers sponsor clinical trials to generate scientific evidence that supports use of their products to gain regulatory approval, and to use in commercial materials to promote drug sales. Previous research has found industry sponsored research may use dubious research practices to generate findings that justify use. Three examples of such research practices include inappropriate use of enriched enrollment trial design, ghost authorship, and overstatement of research findings.
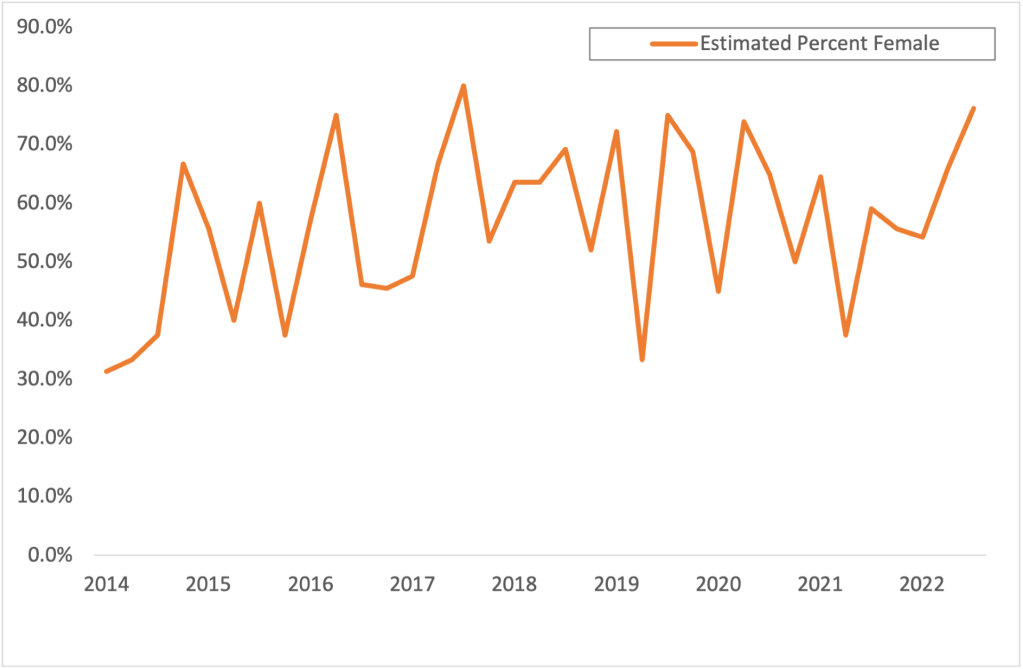
In our recently published Evidence and Policy article, we identified research practices used in clinical trials funded by opioid manufacturers that created the perception that opioids were safe, non-addictive and effective in treating pain. Since 2005, confidential documents made public in litigation against pharmaceutical companies have been collected in the Opioid Industry Document Archive (OIDA) at the University of California San Francisco for storage in perpetuity. In January 2020, OIDA made available the first 503 documents that later become part of the larger OIDA, totaling over 62,000 pages, that were released as part of the Oklahoma litigation in a discrete collection named the Oklahoma Opioid Litigation Documents. These documents included clinical trial reports, witness declarations, internal corporate communications and marketing strategies regarding opioids, and served as the primary data source for the study.
Continue reading